The FSMA aims to reduce foodborne illnesses through better inspection and enforcement of existing regulations. It also provides funding for research and development of new technologies to enhance food safety.
- What reasons for applying (the FSMA) system?
- Summary
- Frequently Asked Questions (FAQ)
What reasons for applying (the FSMA) system?
FSMA was passed in reaction to a string of illnesses. Foodborne illness outbreaks and contamination episodes, both domestic and imported demonstrated the need to update the country’s food safety system.
Congress. wide consumer and corporate support approved it, reflecting their shared belief that everyone would benefit from a healthy environment.
A modernized food safety system that decreases food-borne illness and boosts public trust in the food supply ensures the safety of our food and avoids costly food supply interruptions
To achieve its goals, FSMA requires an overhaul and expansion of the FDA’s current food safety program and authorities, as well as a historic shift away from reacting to and solving problems after they happen and toward preventing food contamination in the first place, preventing human illness.
food safety standard
They address four key kinds of pollutants that have the potential to cause harm in FDA rules and advice.
Chemical, biological, physical, and radiological adulteration of meals FSMA Section 110 directs FDA will produce a report to Congress on the need for new regulations and recommendations for the food industry
In terms of human and animal food safety, there are two sorts of standards:
- The production or processing system, environment, and controls that must (or should) be in place (e.g., current good manufacturing practices [CGMP], hazard analysis and critical control point [HACCP] controls, and other preventative measures) are referred to as “conditions of manufacture.”
- “Product standards” are usually numerical and show the highest level of a chemical (e.g., contaminant, additive, or drug residue) that can be present in the final food without causing harm; or specific processing parameters, such as time and temperature restrictions for microbiological risks.
Guidance documents
Guidance documents help businesses comply with regulations by explaining the FDA’s best thinking and giving technical information to facilities with little food safety experience. They encourage constancy.
For example, the FDA just published the fourth edition of the Fish and Fishery Products Regulations.
Hazards and Controls Guidance is helpful to fish processors. By identifying dangers related to their products in building their HACCP plans and assisting them in developing preventive control techniques.
To explain the situation, the FDA will develop similar recommendations on hazard analysis and preventative controls. The two proposed preventative control regulations’ requirements (for human and animal food) FSMA also mandates the produce safety rule.
CHANGES UNDER FSMA
a) Preventive Controls (a) Preventive controls can remove, decrease, or control food dangers, whether they be pathogenic bacteria, chemicals, or other hazards. Congress revised the FD&C Act by adding Section 418 on Hazard Analysis and Risk-Based Preventive Controls as part of the FSMA section 103.
This part requires human and animal food facility owners, operators, or agents to prepare and implement a documented plan that defines and documents how their facilities will perform the hazard analysis.
b) Produce Safety Standards. FSMA section 105 revised the FD&C Act, which added.
Standards for Produce Safety, section 419 This clause mandates the FDA develop science-based minimum guidelines for the safe cultivation and harvesting of those fruits and vegetables.
veggies for which we have defined such guidelines to reduce the risk of serious illness
death or negative health repercussions, FDA is required by FSMA to prepare a produce safety plan.f
FDA regulation requires FDA to evaluate what measures are reasonably necessary to prevent the
introduction of dangers that are reasonably expected
c) Verification of Foreign Suppliers. The FSMA also addresses the safety of imported food by requiring importers to conduct risk-based verification of their foreign suppliers to ensure that the food is produced in accordance with processes and procedures, including reasonably appropriate risk-based preventive controls.
That provides the same level of public health protection as those required under the FD&C Act’s hazard analysis and risk-based preventive controls and standards for produce safety.
d) Performance Expectations FDA is required by Section 104 of the FSMA to determine the most significant pollutants every two years and to set suitable performance criteria for those contaminants as needed.
CHALLENGES FOR THE FUTURE
The guidelines listed above serve as the foundation for the FSMA-mandated modern, prevention-oriented food safety system. Completing these rules and their supporting materials is consequently one of FSMA’s top priorities.
Besides these guidelines, FDA is required to develop standards for safe food transportation and to prevent food adulteration.
The FDA must also complete soon those regulations.
COMMUNICATIONS, OUTREACH, AND TECHNICAL
ASSISTANCE
FSMA recognizes that efficient food safety systems require effective communications, outreach, and technical help, which can serve a variety of purposes depending on the audience. Many of these initiatives are already underway and will:
- Improve the quality and utility of rules by involving stakeholders early in the formulation process so that their perspectives can better inform rule-making.
• Improve compliance by ensuring that the regulated industry understands FDA regulations through training, technical support, and other ways.
• Ensure coordination and consistency with other federal, state, and local public health government departments.
• Inform food handlers along the farm-to-table chain about their responsibility for foodborne illness prevention.
• Keep all stakeholders informed about particular and possible safety threats to food supply security, such as during outbreaks of foodborne illness and food recalls.
Hagan Amendment
Senators Jon Tester and Kay Hagan offered two amendments that removed farmers, ranchers, and local processors from federal oversight, letting them operate under the existing regulatory framework of state and local health and sanitation laws and procedures, as they do now.
The amendment provided safeguards for operations (also known as “qualifying facilities”) that sell less than $500,000 per year and sell the majority (over 50%) of their products directly to consumers within a 400-mile radius of their location.
The change also applies to all operations classed as “tiny businesses” by the FDA. Some criteria and produce safety laws introduced under S. 510 would not be required on small, local farms.
Municipal and state governments would continue to control small-scale producers (such as those who sell their wares at farmers’ markets or roadside booths).
Either through direct sales or explicit labeling, buyers would know who they are buying from.
Impact and fees
From farmers to manufacturers to importers, the legislation impacts every facet of the US food industry. It places substantial responsibility for preventing contamination of farmers and food processors, in contrast to the country’s reactive habit of relying on government inspectors to catch tainted food after the fact
The bill imposes a $500 yearly registration fee on Food Safety manufacturers and importers to help support increased FDA inspections, enforcement, and related operations such as food-safety research. The fees would apply to around 360,000 facilities in the United States and internationally.
According to the Congressional Budget Office, the fees will not cover the cost of the new system, leaving the FDA with a $2.2 billion net cost over five years.
Prevention
The FDA will have a legislative mandate for the first time, requiring comprehensive, science-based preventative measures across the food chain, including pet food and animal feed.
A written Hazard Analysis and Risk-based Preventive Controls (HARPC) strategy is essential for food facilities. This entails:
Mandatory preventive controls for food facilities
- 1) identifying potential threats to food safety,
- (2) stating what preventive measures, or controls, will be implemented to considerably reduce or eliminate the threats,
- (3) describing how the facility will guarantee that these controls are working by monitoring them.
- (4) maintaining routine monitoring records,
- (5) Outlining the steps the facility will take to address any issues that occur.
Mandatory produce safety standards
The FDA must develop science-based minimum requirements for safe fruit and vegetable cultivation and harvesting.
Those guidelines must encompass soil amendments (materials added to the soil such as compost), cleanliness, packaging, temperature controls, animals in the growing area, and water, as well as naturally occurring dangers and those that may be introduced either mistakenly or intentionally. (About 2 years after legislation, the final regulation is due.)
Radiological hazards
For the first time, under chemical safety, companies must expressly consider radioactive contamination as part of their hazard analysis. The FDA does not believe this is a risk that will cause ongoing monitoring with a Geiger counter.
Instead, a company that uses spring water in its goods, for example, should have the water tested for dissolved radon, tritium, and heavy metal pollutants regularly.
Authority to prevent intentional contamination
Authority to prevent contamination by accident The FDA must create regulations to protect against intentional food adulteration, including science-based mitigation methods to prepare and protect the food supply chain at susceptible locations. (We must issue the final rule 18 months after enactment.) This is the first time that we have included Food Defense in the legislation.
Inspection and compliance
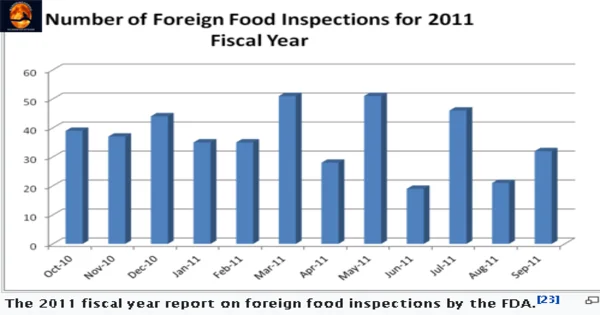
The FSMA acknowledges that preventative control rules only improve food safety if producers and processors follow them. The FDA has expanded jurisdiction under FSMA to conduct inspections and assure compliance.
Mandated inspection frequency The FSMA creates a mandatory inspection frequency for food facilities depending on risk, and it mandates that the frequency of inspections be increased immediately.
Within five years of implementation, all high-risk domestic institutions must be examined, and then every three years thereafter.
The statute requires the FDA to inspect at least 600 overseas facilities within one year of adoption and to double those inspections every year for the next five years.
Because of a lack of resources to satisfy the demand, the USFDA and other US government agencies will form partnerships or collaborate with foreign governments to achieve this predicted target.
Access to records The FDA would have access to documents, including industry food safety plans, and we will compel corporations to preserve records of their plans’ implementation.
Accredited laboratories conduct accredited laboratories and the FSMA orders the FDA to develop a laboratory accreditation scheme to guarantee that food testing facilities in the United States satisfy high-quality standards and must perform testing Certain food testing. (We must establish an accreditation program within two years of enactment.)
Visual examination The FDA will inspect during an unannounced inspection. During the inspection, they will examine the structure and equipment to see if food contamination is a risk. Poor welds, moisture leaks, and open product lines will all be investigated.
Environment swapping During their cursory walk, the agent will seek any spots or niches that they believe could be a bacteria harborage point.
Depending on the size of the facility, the agents can and will take anywhere from 150 to 200 swabs. The agent will also take samples of raw materials and completed goods.
We recommend that the company does not take companion samples because this increases the risk of a lab error and does not seem good if the FDA’s samples are negative, but the facilities are positive, or vice versa.
Response to contaminants/violations
The bill empowers the FDA to issue food recalls in the event of contamination or sickness. It also requires farms to track their food and develop procedures for dealing with recalls or illness outbreaks.
These new laws do not apply to small farms that sell locally or sell less than $500,000 per year. The following new authorities:
A recall is required. When a corporation fails to voluntarily recall dangerous food after being asked by the FDA, the FSMA gives the FDA the right to order a mandatory recall.
Administrative detention was increased The FDA now has a more flexible threshold for administratively detaining products that may violate the law, thanks to the FSMA (administrative detention is the procedure the FDA uses to keep suspect food from being moved).
Registration suspension If the FDA believes that the food poses a real risk of severe adverse health consequences or death, it can suspend a facility’s registration. A facility that is under suspension can not distribute food. (Six months after enactment, it becomes effective.)
We have improved product tracing capabilities. We have given The FDA instructions to develop a system that will improve its ability to track and trace both domestic and foreign foods.
The FDA is required to conduct pilot projects to investigate and evaluate ways to quickly and effectively identify food recipients in order to prevent or contain a food-borne illness outbreak. (We must implement pilots within 9 months following enactment.)
Additional Keeping Track of High-Rise Foods The FDA has been directed to produce a proposed rulemaking to create record-keeping requirements for facilities that manufacture, process, pack, or store goods designated as high-risk food-safe by the Secretary. (Implementation is due two years following passage.)
Employee protections
Someone likewise protected employees who endeavor to prevent food safety hazards under the FSMA. Employers taking part in the manufacture, processing, packing, shipping, distribution, reception, holding, or importing of food are prohibited from retaliating against employees who report violations of the Federal Food, Drug, and Cosmetic Act under Section 402 of the FSMA. The United States Department of Labor is in charge of this section of the FSMA.
Rules
Consumer groups, the Center for Food Safety (CFS) and the Center for Environmental Health sued the FDA in 2012 for failing to meet deadlines. The government agreed to timelines for several rules in 2015 and 2016 as part of the settlement.
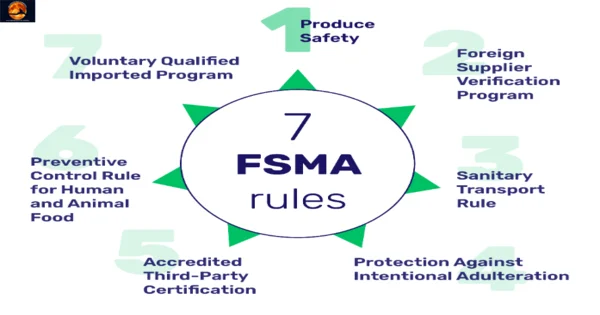
- FSMA rule: Produce safety rule.
- FSMA rule: Foreign supplier verification program (FSVP)
- FSMA rule: Sanitary transportation rule.
- FSMA rule: Protection against intentional adulteration.
- FSMA rule: Accredited third-party certification.
- FSMA rule: Preventive control rule for human and animal food.
FSMA progress report
The FDA is now planning to make considerable progress in implementing the FSMA available to the public and Congress. Sherri McGarry, Senior Advisor, Coordinated Outbreak Response and Evaluation Network, FDA, announced the foods to be utilized in the pilot experiment on tracing items to avoid infections on a blog in March 2012.
Tomatoes, frozen Kung Pao-style foods, jarred peanut butter, and dry, packed peanut/spice are also on the list.
Tomatoes, both sliced and whole, were chosen because of the large number of outbreaks reported; it reflects a complex food supply chain, and we identified it as the number one food product to be used in the pilot program by most of the food industry organizations.
Funding
Along with the Government Performance and Results Modernization Act of 2010, we passed the Safety Act into law. We expect the first five years to cost $1.4 billion and are not yet fully funded.
Food facility registration
The FDA’s revised food facility registration system has been available since October 22, 2012.
All establishments that were previously registered before October 1, 2012, must now renew their registration. Failure to do so is illegal, and it will cause the rejection of foreign items and the illicit trading of domestic facilities.
The most significant words
food safety and hygiene
Food hygiene is a set of food manufacturing practices designed to minimize biological food hazards through safe and clean operations in order to protect public health from foodborne illness.
Food safety is a management system used by a food business to ensure that hazards are controlled to acceptable levels. Food safety addresses all types of hazards and includes a system of corrective action, monitoring, and achieving safe operations.
Proper training and seminars can help achieve a higher level of food sanitation for your food business.
food safety regulation
Are a scientific discipline that describes the handling, preparation, and storage of food in a manner that prevents foodborne illness. It includes a set of routines that should be followed to avoid potentially serious health hazards.
food-safety system
An FSMS is a systematic approach to managing food safety hazards in a food business to ensure that food is safe to consume. All businesses are required to establish, implement, and maintain an FSMS based on the principles of Hazard Analysis Critical Control Point (HACCP).
food safety laws
The national regulatory framework is an important pillar of an effective food control system. In all countries, food is regulated by a series of laws and regulations that set out government requirements for food chain businesses to ensure food safety and quality.
The term “food law” refers to legislation that governs the production, trade, and handling of food, thus covering the regulation of food control, food safety, quality, and relevant aspects of food trade throughout the food chain, from the provision of feed to the consumer.
state food safety
StateFoodSafety is an online food safety training company dedicated to educating the public about food safety and helping to ensure the health of communities across the country.
StateFoodSafety’s training and certification programs are based on industry-leading technology and best practices in food safety.
Summary
Every even-numbered year, between October 1 and December 31, every registered facility must renew its registration. The FDA food facility registration website accepts registrations via fax, mail, and electronic means. The FDA had 195,518 food facilities registered as of January 22, 2014.
Is revolutionizing the nation’s food safety system by focusing on avoiding food-borne illnesses rather than responding to them.
The Food Safety Modernization Act (FSMA) was enacted in response to significant changes in the global food system and our understanding of foodborne illness and its consequences, including the realization that preventable foodborne illness is both a significant public health problem and a threat to the food system’s economic well-being.
The FDA has adopted seven important guidelines to implement FSMA, acknowledging that food safety is a shared responsibility across many various places in the worldwide supply chain for both human and animal food.
We intend the FSMA standards to spell out the particular steps that must be taken to prevent contamination at each of these locations.





